COVID-19 Rapid Antibody Test Kits
The first step to a re-opening strategy is making sure your people are safe.
You’ve probably been reading about states seeking to re-open following the COVID-19 pandemic. But, America can’t get back to work until we have a test to determine if people are recovered or immune from the disease.
There is a lot of misinformation circulating about antibody tests right now. People don’t understand what they are, how they work or how they are to be used. We would like to not only help provide your business with the rapid antibody test kits, but also help guide you through the conversation so you can be knowledgeable and secure in your decision making.
HEALGEN Rapid Antibody Detection Kits
This rapid test kit is intended to screen patients for COVID-19. In general, antibodies can be detected 1-3 weeks after infection, but the test itself can deliver results in as little as 2-10 minutes. Plus, you can be assured that the test is accurate. Combining RNA and antibody tests can significantly raise the sensitivity for detecting COVID-19 in infected individuals.
- Fast results as soon as 2-10 minutes
- Simple, time-saving test procedure
- Small testing specimens (uses a single finger prick)
- No additional equipment needed
- High sensitivity and specificity
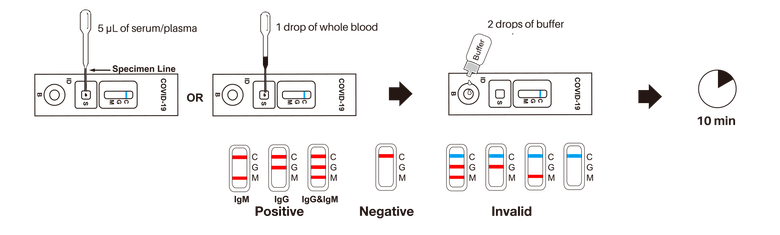
How to Read the Test
The Rapid Antibody test provides differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies. This means you can determine who has recovered from COVID-19 but is producing the antibodies and still potentially contagious (IgM). You can also see who has fully recovered, is no longer contagious and has the antibodies present (IgG).
Learn More:
- Clinical Study of Test Sensitivity & Specificity
- Rapid Antibody Test Fact Sheet
- Rapid Antibody Test Instructional Insert
- FDA FAQs on Diagnostic Testing for COVID-19
How to Get Your Rapid Antibody Test Kits
Disclosure:
This test has been validated, but independent review by the FDA is not yet complete.
This product is intended for professional use and not for home use. Results from antibody testing are presumptive and should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
A negative result may appear and not correlate with symptoms. A medical professional is required to consider various factors, including confirmation testing, when receiving a presumptive result.

